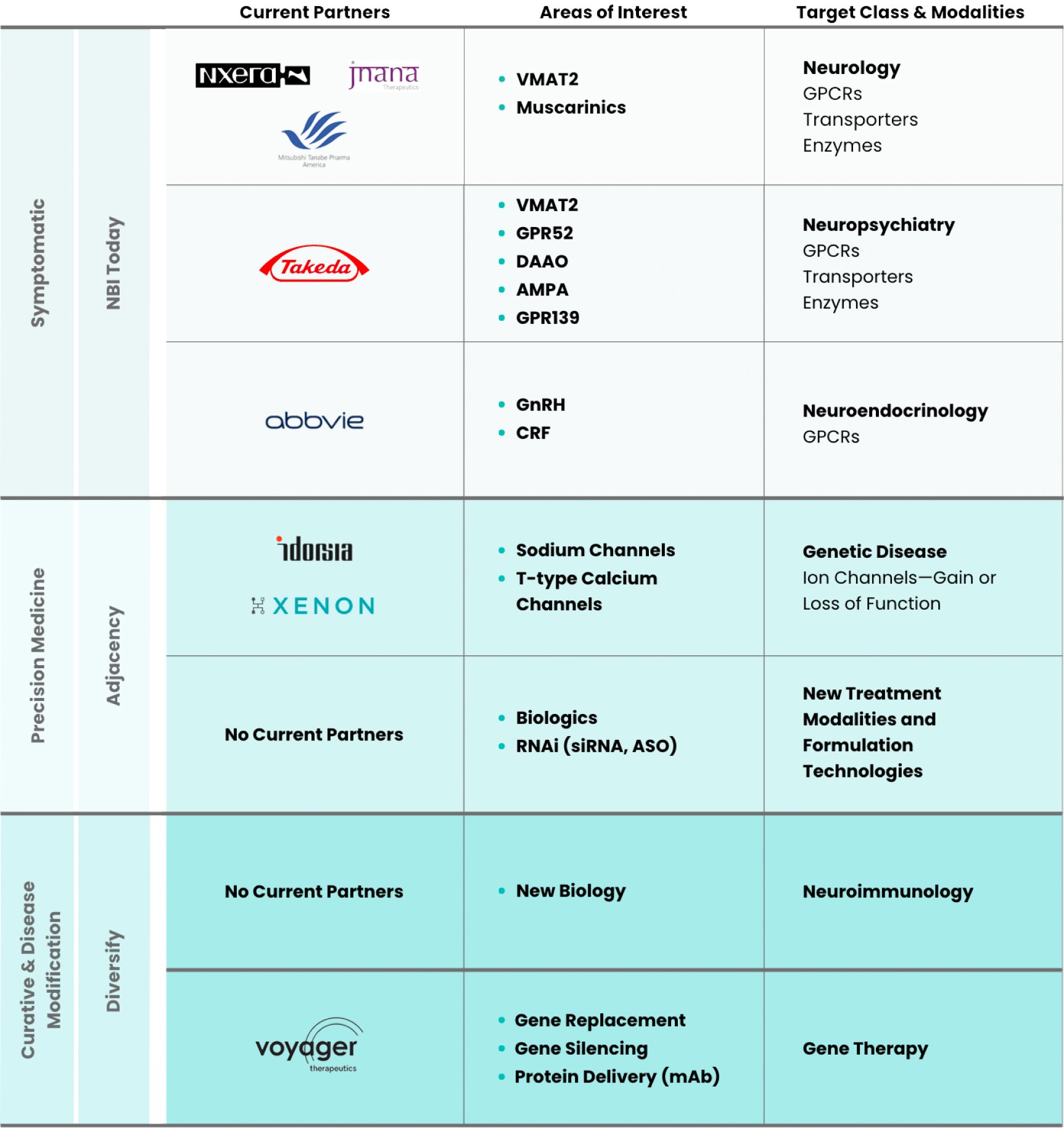
License and collaboration agreement to identify, research and develop Na channel inhibitors.
Read MoreExclusive worldwide collaboration to develop and commercialize elagolix for women's health.
Read MoreResearch collaboration for discovery of small molecules for target associated with CNS disorders.
Read MoreStrategic collaboration focused on the development and commercialization of Voyager Therapeutics' gene therapy programs.
Read MoreStrategic collaboration and licensing agreement to develop novel muscarinic agonists.
Read MoreAcquisition and global rights to NBI-827104, a T-type Ca channel blocker, and a research collaboration to discover and identify novel T-type Ca channel blockers.
Read MoreExclusive collaboration and licensing agreement for the development and commercialization of valbenazine in Japan and other select Asian markets.
Read MoreStrategic collaboration to develop and commercialize early-to-mid stage compounds in Takeda's psychiatry pipeline.
Read More